Fish are prone to hundreds of parasitic and non-parasitic diseases, especially when grown under controlled conditions. Adverse hydrological conditions often precede parasitic attacks, as the resistance of fish is thereby lowered. Mechanical injuries sustained by a fish when handled carelessly during fishing and transport may also facilitate parasitic infection.
The prevalence of fish diseases is very much dependent on the intensity of stocking. So when a farmer decides to raise the stocking rate, he not only has to provide extra food, but also has to take special care to prevent and cure outbreaks of diseases. Diseases are more common in freshwater environments, as it has been found that susceptible freshwater fish are significantly free from disease when grown in slightly brackishwater.
Properly managed ponds usually remain free from disease. Carelessness in stocking and feeding may result in serious parasitism and mortality. Prevention is better than cure. Care should be taken to prevent parasites gaining access to the culture ponds from any nearby infected source. Even though several curative methods are available, treatment is difficult and often impracticable in ponds containing large number of fish.
Preventing the spread of disease by quick removal and destruction of infected fish is probably the most effective method of control. Disease-resistant fish should, as far as possible, be selected for stocking.
Methods for disease diagonsis
Fishes are poikilotherms, hence the environmental impact is more in fishes when compared to warm-blooded animals. The following aspects are useful for the identification of diseased fishes.
1. Disease can be diagonised only in freshly killed fishes and live fishes. If it is late after the death of fish, diagnosis is very difficult due to the chemical changes in the body at normal temperatures.
2. Slime production is more in diseased fishes.
3. After death, the fish settle on the bottom of pond. Then come to the water surface due to the gases produced by chemical changes in the body.
4. Mucus samples should be collected from body surface and gills and examine them under the microscope.
5. Change of body colouration.
6. Abnormal behaviour of the fish.
7. Examine the external features, then go for internal examination.
8. Examine the size, colour and shape of the internal organs like liver, kidney and spleen.
9. Examine the fluid accumulation, hemorrhages and inflammations in the body cavity of fish.
10. Take out the samples from vital organs and go for bacteriology, virology and histological studies.
11. Examine for tumors or swelling in the body.
Types of Fish Diseases
The diseases of fishes are classified as parasitic diseases and non-parasitic diseases.
Parasitic Diseases in Fishes
Parasitic diseases are also called as pathogenic diseases or infectious diseases or communicable diseases. The important parasitic diseases are viral, bacterial, fungal, protozoan, helminthic, annelid and crustacean. The loss of fish production from infectious diseases accounts for about 60% of all diseased cases. Hence, the study of infectious diseases is of primary significance to the development of aquaculture.
The parasites are mainly of two types:
1. Ectoparasites: These are found on the body surface, fins and gills. Ex. Argulus, Lernaea, Ergasilus, leaches.
2. Endoparasites: These are found inside the body. These are further divided into 3 types.
a) Cytozoic parasites: These are found in the cells. EMicrosporidia, Glugia.
b) Histozoic parasites: These are found in the tissues.
c) Coenozoic parasites: These are found in the body cavity or inside the alimentary canal. Ex. Diphyllobothrium, nematodes.
Viral diseases in fishes:
Viruses are transmitted from one host to the other through a structure called virion. Viruses are classified mainly based on external structure, shape, size, capsid structure, RNA and DNA nucleic acids. Viruses cause disease by weakening the host tissue or by forming tumors in the host tissues. There is no treatment for viral diseases, only prophylactic measures have to be taken.
a. Lymphocystis:
Woodcock (1904) identified this disease in fishes. Marine, freshwater and aquarium fishes are susceptible to this disease. Turnor formation is the important character of this viral disease. The external lesions are raised, and made up of the growing of granular, nodular tissue which is composed of many greatly enlarged host cells. Matured lesions may become slightly hemorrhagic. Within 6-15 days of infection the tumors grow to 50 thousand times. It caused a lot of damage in the Baltic Sea area in America.
b. Viral Hemorrhagic Septicemia (VHS):
This disease is caused by an unequal shaped fish virus with RNA. This disease occur in salmon fishes. Transmission of the disease occurs through the water by a flagellate. This disease is also called as infectious kidney swelling and liver degeneration in German and pernicious anaemia, infectious or entero-hepatic renal syndrome in France. The symptoms are kidney swelling, reduced appetite, obvious distress, erratic spiral swimming, multiple hemorrhages in skeletal muscles, change in body colour, reddish fins. The only control measure is prevention.
c. Infectious Pancreatic Necrosis (IPN):
This disease is found in trouts. This disease causing high mortality of fry, fingerlings and occasionally larger fish. The symptoms are darkening distention and at time, hermorrhages in ventral areas including bases of fins. There is pronounced pancreatic necrosis. 200 ppm. Of chlorine is effective for treatment.
d. Infective Haemopoitic Necrosis (IHN):
IHN was observed for the first time in trouts in British Columbia (Canada) in 1967. Necrosis is observed in the haemopoitic tissue of kidney in infected fish. This disease occurs more in fry and fingerlings, and occasionally in adults. The symptoms are pale gills, reddish fins, black colouration of the body, abdomen swelling, and huge mortality. The symptoms are clear in 12-45 days after the entry of virus into the host body.
e. Chinook disease:
A small size virus is responsible for this disease in Chinook salmon (Oncorhychus tshawytscha) fingerlings. The symptoms are exophthalmus, distended abdomen, a dull red areas on the dorsal surface anterior to dorsal fin. The liver, spleen, kidney, gills and heart are pale. The disease is transmitted by the egg from the carrier female. No treatment.
f. Channel cat fish virus disease:
This disease occurs in fingerling of cat fish (Iactalurus punctatus). The symptoms are that the fish show abnormal swimming and rotating, hemorrhagic areas on fins and abdomen, fluid accumulation in abdomen and pale gills. There is no treatment for this disease. Destruction of infected fish may prevent spread of the disease.
Bacterial diseases in fishes:
Bacteria are responsible for many fatal diseases in fishes like furunculosis, columnaris, fin or tail rot, vibriosis, dropsy, cotton mouth disease and tuberculosis.
a. Furunculosis:
Furuculosis disease is caused by Aeromona salmonicida in salmon fishes. It is a non-motile, gram-negative bacterium. This disease frequently appears to infect fishes living in the dirty waters containing a large amount of decaying matter. This disease is also observed in few other fishes. The first symptoms of this disease is appearance of boil-like lesions. Others symptoms are blood-shot fins, blood discharge from the vent, haemorrhages in muscles and other tissues and necrosis of the kidney. Bursting of boils allow the spread of this disease among other fishes and also offer suitable areas for fungus growth. In acute forms it is systemic bacterial infection, a septicemia with bacteria present in the blood, all tissues and lesions. Fishes severely infected with the bacteria die in good number.
Remove the severely infected fishes from the pond and supply food containing antibiotics like sulphonamides or nitrofurans. Sulfonamides like sulfadiozine or sulfaguanidine are given orally with food at the rate of 22 gms/100kg. Of fish/day. Other antibiotics like chloromycetin and tetramycin are most effective at a dose of 5-7.5gm/ 100kg of fish/day. Disinfect the eggs with 0.015% solution of metthiolate or 0.185% acriflavin.
b. Columnaris disease:
Columnaris disease is caused by Chondroccus columnaris and Cytophaga columnaris in many freshwater aquarium fish. It is a long, thin, flexible, gram-negative slime bacterium (myxobacteriales). This disease is often associated with low oxygen level. Initially it is marked by appearance of grayish-white or yellowish-white patches on the body. The skin lesions change to ulcerations and fins may become frayed. Gill filaments are destroyed and eventually lead to the death of the fish.
Addition of 1 ppm copper sulphate in the pond to control this disease is effective. Tetramycin administered orally with food at a rate of 3 gm/100 pounds of fish/day for 10 days is very effective. Dip treatment in malachite green (1:15000) for 10-30 seconds and one hour bath in 1 ppm furanase is very effective to control this disease.
c. Fin or tail rot:
Tail or fin rot disease is caused by Aeromonas salmonicid and A.liquefaciens. However, protozoans and fungi may also be involved. It is characterized by appearance of white lines along the margins of fins, the opacity usually progresses towards the base eroding them, and causing hemorrhage. The fin rays become brittle first and later break, leading to the complete destruction of the fins. The infection may also spread on the body surface. Fin and tail rot are associated with poor sanitary conditions in fish ponds and with water pollution in nature.
The fin or tail rot may be checked at an early stage by keeping fishes in 0.5% copper sulphate solution for 2 minutes. Control may be achieved with 10-50 ppm tetramycin and 1-2 ppm of benzalkonium chloride. In severe infections the affected parts are surgically removed and the fishes are then kept in 0.04% potassium dichromate.
d. Vibriosis:
Vibrio bacteria are the causative agents of vibriosis disease in salmon and many other fishes. This disease may occur in waters with low oxygen. These bacteria are small gram-negative bacilli, characteristically curved. Diseased fishes show large, bright coloured, bloody lesions in the skin and muscles, hemorrhages in eyes, gills may bleed with slight pressure, and inflammation of the intestinal tract. Sulfamethazine at a rate of 2 gm/100 pounds of fish / day gives good results. 3 – 4 gm/100 pounds of fish/day for 10 days of terramycin also give satisfactory results.
e. Dropsy:
Pseudomonas punctata is the causative agent of this disease. It is characterized by accumulation of yellow coloured fluid inside the

a) Cotton wool disease
b) Tail rot
c) Ich diseased
d) Boil disease
e) Dropsy disease
f) Costiasis
g) Cotton mouth disease
h) Dactylogyrosis
i) Nematode infection
j) Leech infection
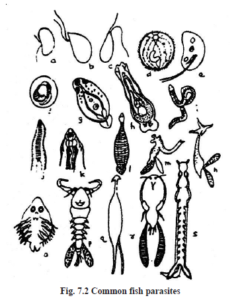
a) Achlya
b) Aphanomyces
c) Saprolegnia
d) Ichthyophthirius
e) Costia
f) Trichodina
g) Diplostomum
h) Dactylogyrus
i) Ligula
j) Philometra
k) Camallanus
l) Hemiclepsis
m) Clavellisa
n) Lernaea
o) Argulus
p) Ergasilus
q) Larnaenicus
r) Caligus
s) Pseudocyonus
body cavity, protruding scales and pronounced exopthalmic conditions. This is known as intestinal dropsy. In case of ulcerative dropsy, ulcers appear on the skin, deformation of back bone takes place and show abnormal jumping. This is a fatal disease in culture systems.
Removal and destruction of fishes, followed by draining, drying and disinfecting the pond with lime are preventive measures to control the disease. The infected fishes may be cured with 5 ppm potassium permanganate for 2 minutes dip bath. Streptomycin and oxytetracyclin give good results.
f. Cotton mouth disease:
The filamentous bacteria, Flexibacteria is the causative agent of this disease. The main symptom is appearance of fungus like tuft around the mouth. This can be treated with antibiotics like 10 ppm chloramphenicol for 2-5 days and 0.3 ppm furanace for long term bath (Fig.7.1).
g. Tuberculosis:
Mycobacterium is a disease causing agent which is difficult to diagnose without pathological examinations. The symptoms are ulcers on body, nodules in internal organs, fin or tail rot, loss of appetite and loss of weight of fish. This can be cured with dip treatment in 1:2000 copper sulphate for 1 minute for 3-4 days. Antibiotics are not successful. The fishes should be destroyed and potassium permanganate or lime used in the pond.
h. Bacterial gill disease:
This disease is caused by Myxobacteria in salmon fish. Many bacteria are found in swollen gill lamellae which show proliferation of the epithelium, and symptoms are lack of appetite. This disease is transmitted through water from infected fish. It can be treated with 1-2 ppm timsan or 1 ppm copper sulphate.
Fungal diseases
a. Saproligniasis:
This disease is also called as cotton wool or water mould disease. This disease is caused by Saprolignia parasitica. It is the most common fungus affecting fishes, especially major carps. The fry and fingerlings, when transported over long distances get bruises on the body, and unless properly disinfected, become sites of infection, resulting in large scalemortality.Whenever fish get injuries the fungal infection may occur. The infected fish becomes weak and lethargic or exfoliation of the skin followed by hemorrhage, exposure of jaw bones, blindness and inflammation of liver and intestine. This can be treated with 1-3 ppm malachite green for one hour or 1:500 formalin for 15 minutes.
b. Branchiomycosis:
This is also called as gill rot. This disease is caused by Byanchiomyces demigrans and B.sanguinis. It is reported to be common on cultivated fishes in ponds having abundant decaying organic matter. The tubules of fungus grow into the respiratory epithelium of the gills, causing inflammation and damage to their blood vessels. The blood supply is stopped to the infected area, as a result of which it becomes necrotic. It can be controlled with 5% common salt for 5-10 minutes.
c. Ichthyophonosis:
It is also known as reeling disease. It is characterized by swinging movement of the infected fish. It is caused by Ichthyophonus hoferi. It enters into the host along with the food. The spores spread to the various organs and in severe cases spread out to the skin which may rupture and become ulcerative at several places. It is extremely difficult to control this disease. The infected fishes are isolated from the stock and kept for treatment in separate ponds. Medicines like sulfamethanis, terramycin, erythromycin and calomel are useful to treat the infected fish.
Protozoan diseases
a. Whirling disease:
This disease is caused by a myxosporidian protozoan, Myxosoma cerebralis only in salmon fishes. The symptoms are pancreatic necrosis, lesions and disintegration of the cartilaginous skeletal support of the organ of equilibrium. Rapid tail-chasing type of whirling is often seen when the fish is frightened or trying to feed. The typical symptoms usually appear at 1-2 months after exposure to the disease. If the pond contains all infected fish, it is better to destroy them by deep burial. Then the pond should be cleaned thoroughly and disinfected with calcium cyanamide, quick lime or sodium hypochlorite.
b. Costiasis:
This is caused by a mastigophore, Costia necatrix in culture fishes. This is a common disease in ponds where fishes live densely in water with a low pH and poor condition food. The parasites live in large numbers on fish skin, fins and gills. The symptoms are appearance of grey blue film on the skin, which turns to red patches in severely affected cases. The infected fish becomes weak, loss of appetite occurs and finally die. They can be treated with 3% common salt for 10 minute or 1:2500 formalin solution.
c. Ichthyophthiriasis:
This caused by a ciliate, Ichthyophthirius multifilis. This disease is also called as ich or white spot disease. The young parasites moving in water get attached to the skin of the fish. They grow between the epidermis and dermis and after becoming large in size fall to the bottom of the pond. Infected fish develop small white spots on the skin and the fins. These parasites attack the gills also. Fish respond by jumping in the water and rubbing their body against the water objects. Respiration gets affected and they finally die. Dip treatment in 1.5 ppm of malachite green or in 10 ppm of acriflavin gives good results. 3% salt solution, 1:4000 formalin, 1:100000 quinine hydrochloride, 1:500000 methyl blue are also useful to treat the fish.
Helminthic diseases
a. Dactylogyrosis:
The monogenic trematode, Dactylogyrus is reported to cause serious infection in fishes. D. exitensis, D. vastator and D. lamellatus are found in carps. These are found on the body, fins and gills. The parasites start appearing in the ponds during the rains, but their prolific multiplication takes place during winter, when the intensity of infection on carp fry may reach as high as 94%. The most infected size group is 61-100mm, irrespective of species. Infected fishes rest near the surface of the pond margin, swim very slowly, feel suffocation, are more slimy, dropping, and folding of fins and pale gills. Alternative baths with 1:2000 acetic acid and 2% sodium chloride are effective. 10 ppm of potassium permanganate bath for 1-2 hours and 5 ppm in the pond may give good results. Bromex – 50 (0.18 ppm) and Dylox (0.25 ppm) are effective to control the disease.
b. Gyrodactylosis:
Another monotreme trematode, Gyrodactylus also causes disease in culture ponds. This also lives on fins and on the body of the fish. The symptoms are production of more slime, damage of fins and fading of the body colour. The medicines used in control of dactylogyrosis are also effective to control this disease.
c. Other helminthes
Like Diphyllobothrium, Bothriocephallus, Diplostomum, Clinostomum, and spring headed worms (Acanthocephala) cause diseases in fishes. Nematodes also cause diseases in fishes of which some of the common nematodes are Phillometra and Camallanus.
Leeches diseases
Leeches belonging to the gnathobdella and rhynchobdella attack the fishes. Leeches like Piscicola, Myzobdella and Hemiclepsis hold the skin of the fish and suck fish blood. After the blood meal they detach themselves, leaving the wound open for secondary fungal infections. The growth of fish is affected and they become weak. A popular control method is dip treatment in 2.5% sodium chloride for 30 minutes. This helps to detach the parasite from the body of the host. Use 1 ppm dylox for 5 days. Remove the infected fishes from the pond for treatment, and drain and disinfect the pond with lime to destroy the eggs and adult leeches.
Crustacean diseases
a. Argulosis:
Argulus or fish lice is a common copepode parasite in fishes. It is a large ectoparasite and can move over the body surface of the fish. Argulus puncture the skin and inject cytolytic toxin through the oral sting to feed on the blood. The feeding site becomes a wound and hemorrhagic, providing ready access to secondary infection of other parasites, bacteria, virus and fungi. Argulus transmits dropsy in fishes. In advanced stages, fish swim erratically, show growth loss and loss of equilibrium.
To control Argulus, remove the submerged vegetation, wooden lattices placed in the pond will serve as artificial substrate to deposit its eggs, which can be removed at intervals to kill the eggs. 500 ppm of ammonium chloride, 410 ppm of balsam, 10 ppm of DDT for 25 seconds dip, 0.25 ppm of dylox and 2000 ppm of Lysol for 15 second dip are effective to kill Argulus.
b. Lernaeasis:
It is caused by a copepode parasite, Lernaea or anchor worm. This disease is mostly caused by L.cyprinacea. The larval stages are temporary parasites that feed on mucous and blood of fish. The adult female is a specialized fish parasite, worm like, which burrows into the fish flesh, keeping its eggs cases protruding out of the fish body. Male Lernaea do not attack the fish and are not specialized for parasitic life. Early infected fish swim erratically, flashing against the sides and bottom of ponds. Heavily infected fish swim upside down or hang vertically in the water.
Only partial control of Lernaea is possible with chemicals, because the head is buried in the fish tissues and there are no exposed respiratory organs. Hence, prevention is more effective than control. 1% common salt eliminates larvae in 3 days, 250 ppm formalin for 30 to 60 minutes. 0.2 ppm gammexane for 72 hours, 2 ppm of lexone, 0.1 ppm lindane for 72 hours and 1 ppm chlorine for 3 days may give good results.
c. Ergasillus and salmincola:
These two parasites are responsible for huge mortality of fishes in the culture systems. These two parasites are found attached to the gill filaments and feed on blood and epithelium. Later they may also be found on the fins and body. The infection results in impaired respiration, epithelial hyperatrophy, anaemia, retarded growth, restlessness and finally death. The fish becomes susceptible to secondary infection, especially fungus.
Ergasilus can be treated successfully with a combination of 0.5 ppm copper sulphate and 0.2 ppm ferric sulphate for 6 to 9 days. Salmonicola can be controlled with 0.85% calcium chloride, 0.2% copper sulphate, 1.7% magnesium sulphate, 0.2% potassium chloride and 1.2% sodium chloride for 3-4 days.
Achtheres is a common parasite attached to the gill rakers of fishes, but does not damage gill filaments. It can also be controlled by the above chemicals.
Algal disease:
Cyanophyceae member, Oscillatoria is responsible for fish mortality. It is found on gills and fish body in large numbers and produce toxic substances, which are responsible for fish kill. Chlorella and Pharmidium also cause discomfort in fishes.
Epizoic Ulcerative Syndrome (EUS):
Epizoic Ulcerative Syndrome, popularly known as EUS, has caused severe damage to India’s aquaculture, especially at the moment when the Indian fisheries industry is poised for a great leap forward with high input based hitech production systems. Widespread outbreaks of the disease, occur suddenly and often cause mass mortality in freshwater and brackishwater fishes causing anxiety and tremendous concern. Although the disease has been known in the Asia-Pacific region since the seventies, it appeared for the first time in India in 1988 and has now covered almost the entire length and breadth of the country. Barring a few states like Jammu and Kashmir, Punjab, Himachal Pradesh and the Union Territory of Delhi, the disease has been reported from every state by now.
One common feature of the disease is that it initially affects the bottom-dwelling species like murrels, followed by catfishes and weedfishes. Subsequently, the Indian major carps also get affected. There is a growing concern about the disease now since it has also been found to affect several species of fishes in brackishwater bodies like Chilka lake and estuarine waters of Paradeep of Orissa. Auari and Mandovi estuaries of Goa and Vembanad lake of Kerala.
Unlike other diseases, this syndrome has been disturbingly found to affect a variety of fish species, both wild and culturable, resulting in large scale mortalities. The most severely affected ones are Channa sp., Puntius sp., Clarias batrachus, Heteropneusters fossilis and Mastacembelus sp., Other species which are affected are Glossogobius sp., Trichogaster sp., Gadusia sp., Amphpipnous cuchia, Wallago attu, Anaba testudineus, Salmostoma bacaila, etc. Among the major carps, it has been recorded in catla, mrigal, rohu and kalbasu. Common carp, grass carp and silver carp are also affected.
Among the brackishwater fishes, it has been seen in Mugil subvirdis, M. cephalus, Liza bornensis, Etrophus suratensis and Channa striatus. Fishes of all sizes are affected. However, the incidence of infection is more in the younger ones.
Clinical signs and gross pathology in the affected fishes are similar in almost all the species with moderate to severe ulcerative skin lesions. The lesions start as small grain to pea-sized hermorrhagic spots over the body which ultimately turn into big ulcers of the size of a coin, with grayish, slimy central necrotic area surrounded by a zone of hyperemia. The disease affects the fish to such an extent that they start rotating while still alive, and eventually die.
Affected fishes with mild lesion may not show any clinical sign, whereas those with marked ulcerative lesions exhibit distinct abnormal swimming behaviour with frequent surfacing. The internal organs of most of the clinical and sub-clinical cases do not show any gross lesions. In severe cases, hemorrhages have been noticed over the surface of the liver and kidney. Clinical symptoms can be categorized in three stages: 1) Initial stage characterized by localized hemorrhages on scale pockets, 2) Advanced stage showing sloughing off of scales with degeneration of epidermal tissue and the ulceration, and 3) Final stage characterized by deep and large ulcers on various parts of the body.
Till date, several methods have been tried or are being tried to control the disease. Many antibiotics, sulfonamides, herbal preparations and chemicals have been advocated as preventive and curative measures. Yet, lime is the most accepted therapeutic agent. These reagents which help in controlling this disease to some extent are either costly in their application and are not favoured by the farmers who are generally poor.
The success of any developmental planning depends on the identifications of anticipated maladies and provision of suitable remedies. Ultimately, they have been successful in formulating a chemical mixture which has proved to be very effective as a curative as well as a preventive measure against EUS. The chemical mixture has gained immense popularity and affordable price. This mixture has been named Cifax. The yellowish brown liquid is advised to be diluted in a sufficient quantity of water before being sprayed over the waterbody evenly for a thorough mixing. Appreciable changes are noticed in the affected fish within 3-4 days and marked improvement of the ulcerative condition is noticed within 7 days.
Health management
The principles of fish health management incorporates minimizing stress in cultivated fishes, confinement of disease outbreak to affected ponds and minimizing losses from disease outbreak. This could be achieved through prophylaxis and positive treatment to the outbreak of epidemics. Because of the aquatic ambience, it is not easy to be aware of the activities of fish. It is difficult to conduct a correct diagnosis and timely treatment. This necessitates prevention of fish diseases which is more important than control of fish diseases. This signifies the importance of the statement “Prevention is better than cure”.
i. Prevention of fish disease
a) Importance: It is difficult to identify the appearance of disease in its initial stage on account of the gregarious nature of fish in water which causes difficulties in observation, diagnosis and timely treatment. Apart from this, some effective drugs and measures to cure certain fish diseases are still not known well. Therefore, perfect preventive measures must be taken since this is a key link in fish disease control.
b) General preventive measures: Increasing the internal resistance of fish is important in the prevention of diseases. Therefore, some important points in fish culture should be special attention.
1. Selection of healthy fish seed.
2. Proper density and rational culture.
3. Careful management
4. Qualitatively uniform ration and fresh food.
5. Good water quality.
6. Prevention of fish body from injury.
ii. Abolishing pathogens and controlling its spreading:
Existence of pathogen is one among three factors (host, causative agent and environment) in outbreak of fish disease. To abolish the pathogen and control its spreading the following measures can be taken.
- Thorough pond cleaning and disinfection. Bleaching powder (chlorinated lime) should be applied at the rate of 50 ppm in the pond. It readily kills all the wild fish species, molluses, tadpoles, crabs and disinfects pond soil and water. In nursery and rearing ponds it is desirable to use malathion at the rate of 25 ppm 4-5 days prior to stocking of fish seeds.
- Disinfection of appliances: Nets, gears, plastic wares and hapas should be sun-dried or immersed in a disinfected solution.
- Disinfection of fingerlings and feeding platform: Disinfection with mild concentration of potassium permanganate solution is helpful during the transfer of the fingerling to stocking tanks. The feeding platform can be disinfected by hanging bleaching powder cloth bags with mixture of copper sulphate and ferrous sulphate (ratio 5:2) near the feeding place. When fish come to the feeding place for feeding purpose, their skin will be automatically disinfected.
- Proper feeding: Fixed quality, quantity, time and place has to be followed for proper feeding. Any reduction in quality and quantity and variations in feed application and place may cause not only deficiency disease but also will increase the susceptibility to many infectious diseases.
- Segregation of year class fish population: Brood and older fish may serve as carriers of disease causing organisms without exhibiting any clinical symptoms. To avoid such risk, young fish should be segregated from the brood and older fish.
- Spot removal of dead fish from the pond: Dead and sick fish should be removed as soon as it is located. The daily loss of fish should be recorded to provide valuable insight to the intensity of disease problem.
- Chemoprophylaxis: Effective and inexpensive prophylactic measures against wide range of parasitic and microbial diseases are advisable as chemoprophylaxis (Table.1) Occasional pond treatment with potassium permanganate at the rate of 2-3 ppm and dip treatments with potassium permanganate at the rate of 500-1000 ppm for 1-2 minutes or short bath in 2-3% common salt solution is safe. Some of the chemoprophylactics used in culture practices are given in Table 7.1 Besides, oral administration can be given for preventing systemic infections.
- Immunoprophylaxis: Immunisation programme is gradually emerging as one of the most important measures for preventing infectious disease. Vaccine to combat bacterial diseases of carps are available in developed countries. Vaccine against Aeromonas hydrophila, Plexibacter columnaris, Edwardsiella tarda, ictaluri, Aerononas salmonicida, Yoreinia ruckeri, Vibrio angullaram and several viral pathogens such as IPNV (infectious pancreatic necrosis virus). CCVD (channel catfish virus disease), VHSV (viral hemorrhagic septicemia virus), IHNV (infectious haemopoitic necrosis virus), etc. are being tried on large scale. Serodiagnostic methods that included Fluorescent antibody test (FAT), Enzyme immuno assay (EIA) and passive haemagglutination (PHA) are employed. Study of virus, viral vaccine preparations, incubating temperature and pH are the determining factors for fish cell culture. “Formalin inactivated vaccine” for hemorrhagic septicemia in grass carp is adopted in China.
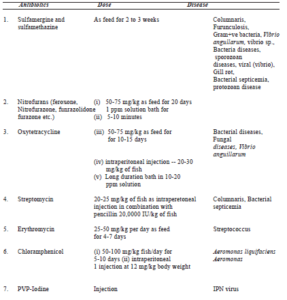
Chemotherapy:
The term chemotherapy was introduced by Paul Ehrlich (1854-1915) cited by Smith, 1967; who was a pioneer in the development of chemotherapeutic agents (Table.7.2). It is a procedure employed to restore normal health condition of fish. Therapy is applied in 3 ways – external treatment, systematic treatment through diet and parentreal treatment.
Table 7.1. Chemoprophylactics



Antibacterial agents or antibactics include Sulfonamides, nitrofurans, furanace, tetracycline. 4-quinolones, erythromycine, chloramphenicol which are being used to combat fish diseases (Table.7.2). In 1941, the term “antibiotic” was defined by Waksman (1946) as a chemical substance produced by microorganisms which have the capacity to inhibit the growth of bacteria and even destroy bacteria and other microorganisms in dilute solution.
Table 7.2. Chemotherapy
Non-parasitic diseases
Non-parasitic diseases are classified into environmental and nutrietional fish diseases.
Environmental Fish Diseases
Environmental diseases are belongs to non-paracitic diseases.
The environment, in which the fish live and grow plays an important role for fish health. Any deterioration in the environmental qualities often creates stress to fish and favour multiplication of pathogens. Though the fish has defensive mechanism against pathogens in the form of scales, epithelial cells, acid and alkali media of alimentary canal, which offers resistance to pathogens, and finally the defense mechanisms regulated by immune system and phage cells, the pathogens predominate and diseases occur in fish farming systems.
Stress response from the environment leads to fish mortality in extreme cases. At sub-lethal level, there may be several other responses like changes in fish behaviour, reduce growth/food conversion efficiency, reduced reproductive potential, reduced tolerance to disease, and reduced ability to tolerate further stress.
The environmental diseases diagnosed are
a) Depletion of oxygen – The mouth remains open. Gills look pale with wide opercle. Bigger fishes die first.
b) Excess of carbondioxide – Excessive secretion of mucus or high pH level in pond by epithelial cells.
c) Nitrogenous waters and ammonia accumulation – Gills look dark red due to formation of methaemoglobin, a combination of nitrogen and haemoglobin.
d) Supersaturation of oxygen or nitrogen – Accumulation of gas bubbles within the body cavity of fish spawn.
e) Excess of hydrogen sulphide gas – Pond muck smells like rotten eggs. The bottom dwelling fish come up to the surface and die first.
f) Organic pollution – Dropping of pectoral fins in case of organo-phosphorus pesticide. Oozing of blood from eyes in some cases.
g) Algal toxicosis – Algal bloom may appear in ponds due to accumulation of plenty of organic matter; or due to excessive chemical fertilizers. Toxins released by blue-green algae like Microsystic, Aanabaena and Aphanizomenon kill other phytoplankton and cause surfacing of fish stock. Persistence of the bloom will cause toxicosis for the fish stock showing symptoms like convulsions leading to death.
h) High temperature of water – The fish on crossing tolerance limit shows the alarm syndrome initially e., coming up to the surface, splashing water and finally exhausted and swimming to the bottom. Indian minor carps die when the temperature is 390C and air breathing cat fishes get exhausted at 420C.
i) Europhication – Water body looks pea-soup green in colour due to bloom of blue green algae.
a. Prevention against environmental diseases:
Proper sanitation by removing muck from pond bottom regularly and exposing the bottom soil to the sun. During summer months, when water level in perennial pond remains at its lowest, lime and potassium permanganate can be used in maintaining sanitation. Liming of ponds has become a must in maintaining sanitation in nursery, rearing and stock ponds. Through restricted use of manure, fertilizer and fish feed, both primary producer (algae) and primary consumer (zooplankton) need to be kept under control, or else the supersaturation or depletion of oxygen will create problems.
b. Acidosis and alkalosis:
A great majority of fish live in pH 7-8. However, if the pH of water goes down drastically owing to reduction of calcium salts or release of humic acids from the soil, a phenomenon known as acidosis results, when the fish may show very rapid swimming movements and a tendency to jump out of water. In the gills of carps, acidosis causes dark-greyish deposits, darkening of the edges and mucous secretion. In the event of mortalities in ponds due to acidosis. The pH must be normalized with powdered calcium carbonate and not with quicklime.
Aquatic plants present in high densities liberate enormous quantities of oxygen during photosynthesis which is responsible for the formation of insoluble calcium carbonate from calcium bicarbonate followed by the formation of calcium oxide with the elimination of carbon dioxide. This phenomenon is known as alkalosis. Excessive alkaline condition leads to the corrosion of bronchial epithelium and fins. Alkalosis can be prevented by buffering the medium by means of suitable calcification. Excessive plant growth in ponds should also be avoided. The lethal acid and alkaline ranges are <4.8 and >9.2 in trout, <5.0 and >10.8 in carps and <4.0 and >9.2 in perches respectively.
c. Gas bubble disease:
When nitrogen of the water is higher than 125 percent saturation due to rapid temperature change, gas bubble disease may result and fish fry particularly, die in large numbers. Fish affected by this disease often swim at an angle of 450 with their head pointing down. Other symptoms are the presence of bubbles beneath the skin, on fins, around the eyes, in the stomach and intestine or in blood capillaries. In such conditions, water should be well agitated to bring down the nitrogen saturation below110 per cent or affected fish should be transferred to other ponds. Besides nitrogen, supersaturated levels of oxygen (>350 percent air saturation) have also been reported to cause gas bubble disease in fishes.
Nutritional disease
Nutritional fish diseases can be attributed to deficiency, excess or improper balance of components present in the food available. Symptoms appear gradually when one or more components in the diet drop below the critical level of the body reserves. Nutrition diseases are presented in Table 7.3.
Table 7.3. Nutritional diseases in fishes
Nutritional components and Symptoms
Protein : Reduce growth rate and body deformities
Carbohydrate : Depress the digestion, symptoms are similar to that of diabetes millitis in warm blooded animals. Enlarge livers. Sikoki disease in carp similar to diabetic symptoms
Lipids : W3 deficiency (linolenic series) causes discoloration, hypersensitivity to shock and large liver. Fat oxidised diet causes muscular destrophy, poor growth. Lipoid liver degeneration is characterised when liver glycogen is replaced by lipoid and ceroid produced from liver lipid Health Management through fat metabolism. Visceral granuloma is due to auto xidation of lipid in diet. Enteritis and hepatoma are due to aflatoxin in diet.
Minerals : Thyroid hyperlasia or goiter caused by iodine deficiency. Dicalcium phosphate deficiency cause scoliosis in carps.
Vitamins (water soluble) :
- Thiamine (vit-B1) deficiency resulted in poor appetite, muscle atrophy, loss of equilibrium similar to that of whirling disease symptoms in trout, odema and poor growth.
- Riboflavin (vit-B2) corneal vascularisation, cloudylens, hemorrhagic eye, photophobia, dim vision, incoordination, discoloration, poor growth and anemia.
- Pyridoxine ((vit-B6) Nervous disorders hyper irritability, aemia serous fluid, rapid gasping and breathing
- Panthothenic acid. Loss of appetite, necrosis and scarring, cellular atrophy, exudates on gills, sluggishness, cubbed gills, poor growth
- Inositol. Fin necrosis anaemia, distended stomach, skin lesions and poor growth.
- Biotin. Blue slime patch on body, loss of appetite, muscle atrophy, fragmentation of erythrocytes, skin lesion and poor growth.
- Folic acid. Poor growth, lethargy, fragility of caudal fin, dark colouration, macrocytic anaemia, decreased appetite
- Choline. Anaemia, hemorrhagic kidney and intestine, poor growth.
- Nicotinic acid. Loss of appetite, photophobia, swollen gills, reduced cooridation, lethargy
- Vitamin (B12) cobalamin derivative. Erratic haemoglobin level, erythrocyte counts and cell fragmentation.
- Ascorbic acid. Lordosis and scoliosis eroded caudal fin, deformed gill operculum, impaired collagen formation.
Fat soluble vitamins
Vit-A – Vit-A causes expthalmos, ascite, odema, hemmorhagic kidney. Hypervitaminosis (A) cause necrotic caudal fin
Vit-D – Necrotic appearance in the kidney
Vit-K – Mild cutaneous hemorrhages due to ineffectiveness of blood clotting
Vit-E – Exophthalmia, distended abdomen, anemia with reduced RBC numbers and haemoglobin content. Accumulation of ceroid in fish liver.
Therapeutic Methods
In recent years, prawns and fishes have gained considerable attention as they form much sought after candidate species in semi-intensive and intensive culture systems. One of the principal factors limiting their productions from natural sources, hatcheries and culture operations have been outbreaks of various disease which cause severe mortalities of the valuable shrimp and fish stock and bring forth considerable economic and production losses. According to a more conservative estimate the farmers of Andhra Pradesh alone have suffered about 500 crores rupees losses by the recent outbreak of white spot disease epizootic of Penaeus monodon in the last quarter of 1994 and first quarter of 1995. These great losses suffered by the aquaculture-industry due to outbreak of diseases underlines the need to focus more attention on this aspect of aquaculture arid to divise suitable trtera-; peutic measures for the treatment and control of shrimp diseases:.
METHODS OF THERAPY IN fish DISEASES
The shrimps are pokilothermic invertebrates. They are highly delicate animals. Any fluctuation in their aquatic habitat cause significant effects on their physiology leading to outbreak of diseases and subsequent mortalities. These variables have a direct bearing on the use of therapeutic agents in combating different diseases. There are many methods of administering therapeutic agents some common among them are as follows
Pond treatment
This technique is frequently used in ponds where shrimps can not be easily removed or concentrated and where the ponds are undrainable. But this method of treatment is effective only in small water bodies, aquaria, cisterns and pools. Moreover, only low concentrations of chernotherapeut i cs can be used as they must be dispersed by natural processes. Acute and advanced diseases can bot be treated effectively by this method as the chemical concentrations are too low to work rapidly. However, this is a very effective method of prophylactic treatment of shrimps for external parasites.
Bath treatment
This method is useful in culture facilities having sions for rapid flow of water. Alternatively, aquaria, sized plastic or aluminium vessels may also be useful and. The bath treatment is essentially of short duration lasting minites to a maximum of one hour only. In this case the re dose of therapeutic agents are mixed thoroughly in the ve<r.s affected shrimps are put into it. Care must be taken to gau stress levels and oxygen depletion due to high population.
Dip treatment
In this method the shrimps are placed in a hand net arid dipped into a concentrated solution of chemotherapeutant for one minute or less. This method has been found highly effective in treatment of acute diseases, but it may cause additional stress on the affected shrimps. Thus’ care should be taken to immediately release them back into the pond water once treatment is over.
Flush treatment
In this technique, the entire doses of the chemical is added at the inlet and allowed to pass through the flow of water into the pond. This method is more applicable in raceways or recircu— latory systems. It has an advantage of using relatively high concentrations of chemicals with virtually no stress due to handling or oxygen depletion. But in this method the distribution of drug depends greatly on the flow pattern of water. Dead spots such as corners may recieve little or no chemical.. Shrimps in those areas are not treated properaly and may die or serve as reservoir of infection.
Constant flow treatment
This method is useful where the water supply is contaminat ed. The shrimps are constantly exposed to pathogens under these conditions and constant presence of drugs may be necessary to prevent outbreaks. Here a constant flow siphon or metering pump is used to monitor the drug to give a constant low concentration of therapeutant. This method is used in ponds having constant flow of water or in large commercial aquaria with recirculatory or running water facility.
Feed treatment
This technique is highly popular tp administer drugs to shrimps for systemic infection. Here, the required drugs -are mixed with the diet and pellets are prepared. The drugs are mixed with the vegetable oil, gelatin or methyl re1lulose and dry feed pellets are coated with it. Mi croencapsulated feed are also prepared combining choiced medications and the same is fed to the affected shrimp. Both these methods prevent leaching of valuable drugs into the water. It should be ensured to have even and uniformly mixing of drugs in the feed and effective utilization of medicated Feed by the affected shrimps. Underdose will be ineffective, while overdose may be toxic.
Paranteral treatment
Paranteral injections are applicable only in the case of valuable broodstock, berried spawners, etc. In smaller size shrimps, it is not practicable. Intramuscular injections ventral-ly in the lower abdominal region can be administered conveniently. Care should be taken to employ small sized needle otherwise it will peirce the whole body and drug may also be leaked. However, the injection method is time consuming and the required handling is highly stressful to shrimps.
Topical treatment
Shrimps and fishes suffer from many external parasite, fungal and bacterial infections, which respond to topical application of drugs. The lesions, ulcers and localized injections of valuable shrimps may be treated with topical application of concentrated chemicals, antiboiotics etc.,
Summary
Fish are prone to hundreds of parasitic and non-parasitic diseases, especially when grown under controlled conditions. Adverse hydrological conditions often precede parasitic attacks, as the resistance of fish is thereby lowered. Mechanical injuries sustained by a fish when handled carelessly during fishing and transport may also facilitate parasitic infection.
The diseases of fishes are classified as parasitic diseases and non-parasitic diseases.
Viruses are transmitted from one host to the other through a structure called virion. Viruses are classified mainly based on external structure, shape, size, capsid structure, RNA and DNA nucleic acids. Viruses cause disease by weakening the host tissue or by forming tumors in the host tissues. There is no treatment for viral diseases, only prophylactic measures have to be taken.
Bacteria are responsible for many fatal diseases in fishes like furunculosis, columnaris, fin or tail rot, vibriosis, dropsy, cotton mouth disease and tuberculosis.
The fungal diseases in fishes are Saproligniasis, Branchiomycosis and Ichthyophonosis.
The protozoan diseases in fishes are Whirling disease, Costiasis and Ichthyophthiriasis.
The helminthic diseases in fishes are Dactylogyrus and Gyrodactylosis.
The crustacean diseases in fhish are Argulosis, Lernaeasis, Ergasillus and salmincola.
Cyanophyceae member, Oscillatoria is responsible for fish mortality. It is found on gills and fish body in large numbers and produce toxic substances, which are responsible for fish kill. Chlorella and Pharmidium also cause discomfort in fishes.
Epizoic Ulcerative Syndrome, popularly known as EUS, has caused severe damage to India’s aquaculture, especially at the moment when the Indian fisheries industry is poised for a great leap forward with high input based hitech production systems. Widespread outbreaks of the disease, occur suddenly and often cause mass mortality in freshwater and brackishwater fishes causing anxiety and tremendous concern.
One common feature of the disease is that it initially affects the bottom-dwelling species like murrels, followed by catfishes and weedfishes.
This syndrome has been disturbingly found to affect a variety of fish species, both wild and culturable, resulting in large scale mortalities. The most severely affected ones are Channa sp., Puntius sp., Clarias batrachus, Heteropneusters fossilis and Mastacembelus.
Therapeutic methods are pond, bath, dip, flush, constant flow and feed treatments
Source : Aquaculture
