Enterocytozoon hepatopenaei (EHP)
- First seen in 2001 in the HP of P. monodon (Chayaburakul et al. 2004. Dis Aquat Org 60:89-96)
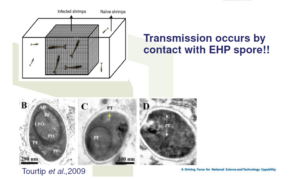
- The disease is called hepatopancreatic microsporidiosis (HPM) (Tourtip et al. 2009. J. Invertebr. Pathol. 102, 21-29.)
Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black shrimp Penaeus monodon (Decapoda: Penaeidae): Fine structure and phylogenetic relationships
• EHP is a species of microsporidia. They are closely related to fungi.
• EHP infections have reached epidemic proportions in Asian shrimp aquaculture.
• Found in Thailand, China, India, Vietnam, Indonesia and probably elsewhere in Asia.
• Causes slow growth in shrimp
• growth retardation is not clearly evident before two months of cultivation

Topics to be presented
• Diagnostic method
• Mode of Transmission
• Virulence mechanism
• Recommendation for control of EHP
Diagnosis of HPM
- HPM shows no clear gross signs to facilitate diagnosis
- When shrimp show severe growth retardation, it may be suspected as a cause
- To confirm diagnosis histological analysis or molecular methods are required
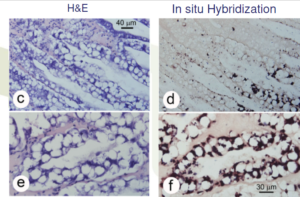
- Histological diagnosis is dependent on finding the presence of EHP spores
- This is difficult by light microscopy because:
– Spores are very small and a 100x lens is required
– Sometimes spores are present in low numbers
Histology is not obvious
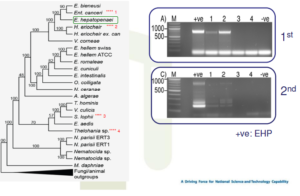
Possible ssu rRNA cross reactions
• Some newly discovered microsporidians have ssu rRNA sequences very similar to EHP
• They infect crabs and fish and other potential carriers of EHP
• Thus, a unique gene of EHP was needed to screen suspected carriers specifically for EHP
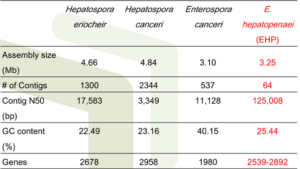
• In cooperating with CEFAS and Exeter University, EHP genome has been sequenced
• As a result, a unique spore wall protein gene specific for EHP was found
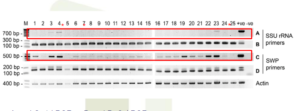
False positive SSU-PCR results for EHP
No false positive results for EHP using SWP-PCR
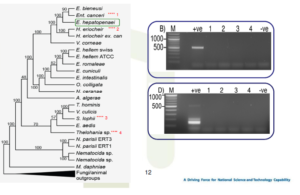
SWP-PCR is better at estimating the relative severity of infections than SSU-PCR
A and C: 1st PCR ; B and D: 2nd PCR
PCR template = total DNA from hepatopancreas of infected shrimp
Suggestions on EHP-PCR diagnosis
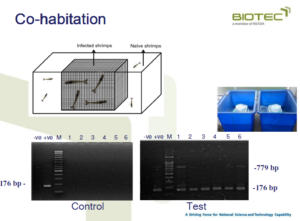
Transmission of HPM

Co-habitation
Cannibalism
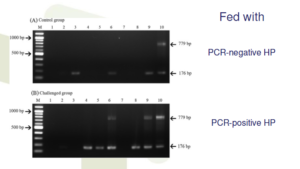
Vertical Transmission was not found
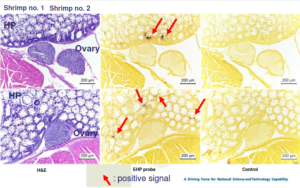
Transmission occurs by contact with EHP spore!!
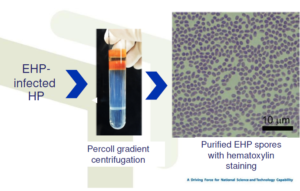
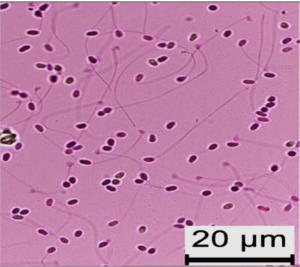
Isolation and purification of EHP spores
Measurement of spore viability
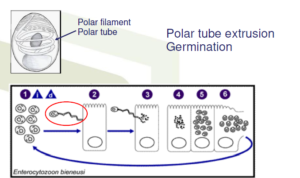
Measurement of spore viability
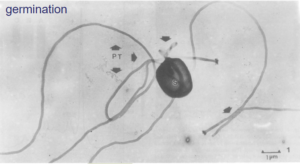
Active purified spores
How can we inhibit spore activity?
Disinfectants
– Chlorine (65% active Chlorine/Ca(ClO)2)
– Formalin
– Potassium permanganate (KMnO4)
Physical
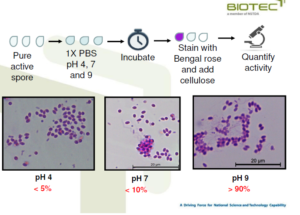
– pH 4/7/9
– temperature 33°C/ 4°C/ -20 °C
Inhibition experiments overview
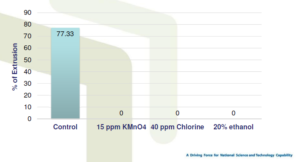
Effect of chemicals on spore extrusion
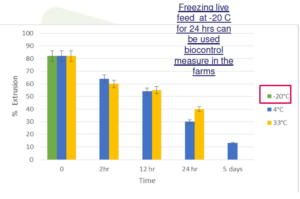
Temperature effect on spore extrusion
Spore activity at different pH
Summary
• Freezing at -20ºC can inhibit spore germination
• 40 ppm of 65% active chlorine,15 ppm of KMnO4 20% ethanol can inhibit spore germination
• Pre-treatment of grow out ponds to increase pH before stocking
• During cultivation, pH should be maintained at pH7-8
Guideline from the laboratory
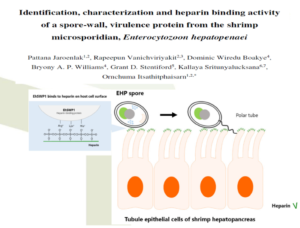
Research on virulence mechanism of EHP

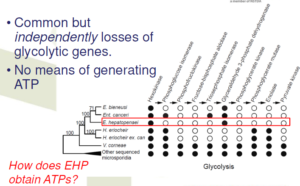
Parallel decay of the carbohydrate metabolisms
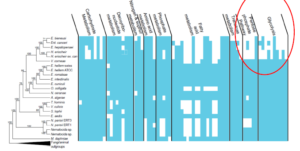
Suggesting a relaxation of the selective pressure to keep glycolysis
Parallel decay of the glycolysis
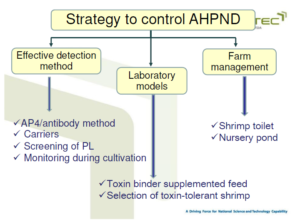
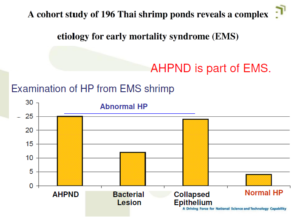
Early mortality syndrome (EMS
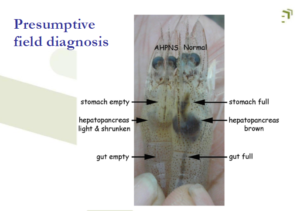
Name for unusually heavy shrimp mortality approx. within the first 35 days of culture
Also called “Acute hepatopancreatic necrosis disease (AHPND)

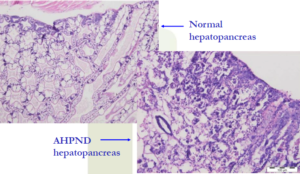
Medial sloughing of HP cells
The key diagnostic feature needed for confirmation
Discovery of AHPND cause
- Specific type of Vibrio parahaemolyticus (VPAHPND)
- First reported from Prof. D. Lightner’s group at U. Arizona (Tran et al., 2013)
- We have found the same isolates in Thailand (Joshi et al., 2013)
- The virulence mechanism of VP AHPND is elucidating.
Bacteria colonize the stomach
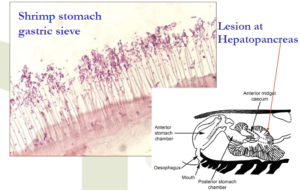
Epidemiology study
• Basic cohort epidemiological study of 200 preselected ponds from EMS areas
• Questionnaires on management and socio-economic issues
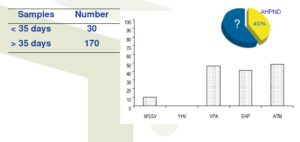
• Ponds with early mortality (<35 days) sampled immediately and sent for multiple analysis
• All other ponds sampled at 35 days and sent for same multiple analysis
Detection of known pathogens and symptoms as Causative Factors of Early Mortality Syndrome (EMS)
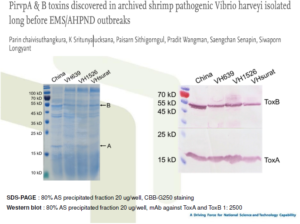
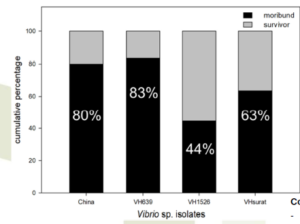
Reverse gavage test
Condition:
– Shrimp: P.vannamei (3 g) = 15 shrimps/test
– Protein: 80% AS precipitated fraction from broth
– Reverse gavage dose: 20 μg protein/g shrimp
– Mortality record: 48 h post reverse gavage

Source: Society of Aquaculture Professionals (SAP)
