Fish seed is the most important component for fish culture. The freshwater resources of our country for fish culture are estimated to be 2.85 million hectares of ponds and tanks. In addition to this, another 2.05 million hectares of water area is available in the form of reservoirs or lakes. It has been estimated that nearly 14250 million fry would be required for stocking even the present available of 2.85 million hectares on a conservative stocking rate of 5000 fry/ha. The present production is 15007 million fry. Apart from this, at least an additional quantity of 4100 million fry are required for stocking the available area of lakes and reservoirs with an average stocking rate of 2000 fry/ha. This indicates that there is a necessity to raise the fry to stock the available water resources.
The fish seed is obtained from three sources – riverine, hatcheries and bundhs. The collection of seed from riverine source was an age old practice. This method is strenuous and we get the mixture of wanted and unwanted fish seed. Hatcheries are the best way of getting fish seed. Apart from these, the bundh breeding is also a good method to collect the fish seed by creating a natural habitat.
The different river systems of India display variations with regard to the distribution and abundance of their fish fauna. This is mainly due to their individual ecological conditions, such as gradient, terrain, flow, depth, temperature, substrata, etc. The northern rivers are perennial and support rich commercial fisheries. Except for the deltaic regions, the fishery of the peninsular rivers is poor both in the upper and middle reaches.
1 Induced Breeding Technology
Carps breed in flowing waters like rivers. Naturally they never breed in confined waters. The seed collected from natural resources is generally a mixed stock with both desirable and undesirable varieties. Separation of desirable seed from mixed stock is a big problem. Due to the handling, the desirable varieties may die. If any predaceous fish seed is found, they injure desirable fish seed. Another big problem is never get required number in natural collection. Availability of pure seed is very difficult. To overcome all these problems induced breeding is an excellent technique to get pure and required fish seed. It has several advantages. With induced breeding pure seed of desirable species can be obtained. Suppose rohu seed is necessary, only rohu seed can be produced in a couple of days. Required number of seed can be produced with this technique. Suppose a fish farm needs 1 crore fish seed, this number can be produced very easily in less time. The problems of identification and segregation of seed does not arise. This technique is very simple. Healthy seed can be produced. Fish can be spawn more than one time in one year. Hybridization is possible.
In induced breeding techniques, four main types of materials are used to give injections to fish – pituitary gland extractions, HCG, ovaprim and ovatide.
Induced Breeding with Pituitary Gland Extraction
Fish breeding by pituitary gland extraction is an effective and dependable way of obtaining pure seed of cultivable fishes and is practiced today on a fairly extensive scale in India as well as many other countries in the world. It involves injecting mature female and male fishes with extracts of pituitary glands taken from other mature fish.
Historical Background:
The present day concept of the role of pituitary in the reproduction of vertebrates is reported to have originated from the experiments of Aschheim and Zondek in 1927 when they found that pituitary implants accelerated the sexual development of female mice. Three years later, in 1930, Houssay of Argentina performed the first such experiment on a fish. He injected a small viviparous catfish, Cresterodon decammaculatus with extracts of pituitary gland prepared from another fish, Prochilodus platensis bringing about the premature birth of developing young. In 1934, a successful technique could be worked out by Von Ihring in certain Brazilian pond fishes were made to spawn by injecting them with a suspension of fresh pituitary glands collected from other less valuable species of fishes. The Brazilians, thus, were the first to use the technique of fish breeding successfully through hypophysation. In 1937, Russian scientist Gerbiskii succeeded in inducing a significant number of sturgeons, Acipenser stellatus.
India is the third country in the world to make the technique an integral part of its piscicultural programme. The first attempt at hypophysation in India was made by Hamid Khan in 1937 when he tried to induce spawning in Cirrhinus mrigala by the injection of mammalian pituitary gland. The next attempt was made by Hussain in 1945 with certain hormones like 80-120 RV Prolan and Antuitrin-S into female Labeo rohita and Cirrhinus mrigala. In 1955, Hiralal Choudhuri succeeded in inducing spawning in Esomus danricus by intraperitonal injection of pituitary extract of Catla catla. He also succeeded in the breeding of Pseudotropius atherinoides. Ramaswamy and Sunderaraj succeeded breeding in Heteropneustes fossilis and Clarias batrachus in 1955 and 1956 respectively. The first success in induced breeding of Indian major carps through hypophysation was achieved in 1957 by Hiralal Chaudhuri and Alikunhi at CIFRI, Cuttack.
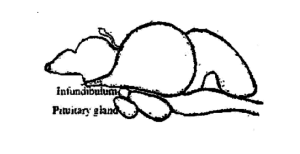
Fish Pituitary Gland:
Fish pituitary gland is a small, soft body and creamish white in colour. It is more or less round in carps. It lies on the ventral side of the brain (Fig. 3.1) behind the optic chiasma in a concavity of the floor of the brain-box, known as Sella turcica and enclosed by a thin membrane called duramater. In few fishes it is attached to the brain by a thin stalk, known as the infundibular stalk. Based on the infundibular stalk, the glands are classified into two types, namely, platybasic – without stalk, have an open infundibular recess and leptobasic – with stalk, have obliterated infundibular recess. Leptobasic type of pituitary glands are found in carps and platybasic type found in channidae and nandidae. The size and weight of the gland varies according to the size and weight of the fish. In Labeo rohita, the average weight of the pituitary gland is 6.6 mg in 1-2 kg fish, 10.3 mg in 2-3 kg fish, 15.2 mg in 3-4 kg fish and 18.6 mg in 4-5 kg fish.
Pituitary gland secretes the gonadotropic hormones, FSH or Follicular Stimulating Hormone, and LH or Luteinizing Hormone. Both hormones are secreted through out the year, but the proportion in which they are secreted is directly correlated with the cycle of gonadal maturity. The FSH causes the growth and maturation of ovarian follicles in females and spermatogenesis in the testes of males. LH helps in transforming the ovarian follicles into corpus lutea in females and promoting the production of testosterone in males. These hormones are not species specific, i.e., a hormone obtained from one species is capable of stimulating the gonads of another fish. However, there is great variability in its effectiveness in different species. Experiments conducted on induced breeding of fishes have clearly shown the relative effectiveness of fish pituitary extracts over mammalian pituitary hormones, sex hormones and various steroids. This is the reason why fish pituitary is being extensively used today in fish breeding work all over the world.
Collection of Pituitary Gland:
The fish donating the pituitary gland i.e., the fish from which the pituitary gland is collected is called the donor fish. The success in induced breeding of fish depends to a great extent on the proper selection of the donor fish. The gland should preferably be collected from fully ripe gravid fishes, as the gland is most potent at the time of breeding or just before spawning. The potency of the gland decreases after spawning. Glands collected from immature or spent fishes usually do not give satisfactory results. Glands in induced-bred fishes collected immediately after spawning have also been found to be effective and can be used for breeding of other fishes. Most suitable time in India for collection of pituitary glands of major carps is during May to July months, as the majority of carps attain advanced stages of their maturity during this period. Since common carp, Cyprinus carpio is a perennial breeder, its mature individuals can be obtained almost all the year round for the collection of glands. The glands are usually preferred to be collected from freshly killed fishes but those collected from ice-preserved specimens are also used.
Several techniques are adopted for the collection of pituitary glands in different countries. In India, the commonly adopted technique of gland collection is by chopping off the scalp of the fish skull by an oblique stroke of a butcher’s knife. After the scalp is removed, the grey matter and fatty substances lying over the brain are gently cleaned with a piece of cotton. The brain thus exposed is carefully lifted out by detaching it from the nerves. In majority of the cyprinids, when the brain is lifted, the gland is left behind on the floor of the brain box. The duramater covering the gland is then cautiously removed using a fine needle and forceps. The exposed gland is then picked up intact without causing any damage to it because damaged and broken glands result in loss of potency.
Glands are also collected through foramen magnum. It is, in fact, a much easier method of gland removal which is commonly practiced by the professionals for mass-scale collection in crowded and noisy fish markets. In this method of gland collection, the fish is required to be essentially beheaded. In markets, glands are collected from fish-heads that are already cut by retailers. In the cut fish-heads, the foramen can be clearly seen from behind holding grey matter and fatty substances in it. The brain lies on the ventral sides of the foramen. For taking out the gland, the grey matter and fatty substances are first removed by inserting the blunt end of the forceps into the foramen and pulling out the entire matter without disturbing the brain. The brain is lifted up carefully and pushed forward or is pulled out of the hole. The gland lying at the floor of the brain box is then picked up using a pair of fine tweezers. An experimental worker easily manages to collect about 50-60 glands in one hour by adopting this technique of collection.
Preservation of Pituitary Glands:
If the collected glands are not meant for use then and there, they must be preserved. Due to their glyco- or muco- protein nature, they are liable to immediate enzymatic action. The pituitary glands can be preserved by three methods – absolute alcohol, acetone and freezing. Preservation of fish pituitary gland in absolute alcohol is preferred in India. Moreover, experiments done so far with alcohol preserved glands on Indian major carps have given more positive results than with acetone preserved glands.
The glands after collection are immediately put in absolute alcohol for defatting and dehydration. Each gland is kept in a separate phial marked serially to facilitate identification. After 24 hours, the glands are washed with absolute alcohol and kept again in fresh absolute alcohol contained in dark colour bottles and stored either at room temperature or in a refrigerator. Occasional changing of alcohol helps in keeping the glands in good condition for longer periods. In order to prevent moisture from getting inside the phials, they may be kept inside a dessicator containing some anhydrous calcium chloride. It is preferable to keep the glands in a refrigerator. They can be stored in refrigerator upto 2-3 years and at room temperature upto one year.
Acetone also is a good preservative. In this method, soon after collection, the glands are kept in fresh acetone or in dry ice-chilled acetone inside a refrigerator at 100 C for 36-48 hours. During this period, the acetone is changed 2-3 times at about 8-12 hours intervals for proper defatting and dehydration. The glands are then taken out of acetone, put on a filter paper and allowed to dry at room temperature for one hour. They are then stored in a refrigerator at 100 C, preferably in a dessicator charged with calcium chloride or any other drying agents. The preservation of glands in acetone is largely practiced in USSR and USA.
Preparation of Pituitary Gland Extract:
Preserved glands are then weighed. This is essential for accurate determination of the dose to be given according to the weight of the breeders. The weight of the gland may be taken individually or in a group. To get a more accurate weight, a gland should be weighed exactly after two minutes of its removal from alcohol.
The pituitary extract should be prepared just before the time of injection. The quantity of gland required for injection is at first calculated from the weight for the breeder to be injected. The glands are then selected and the required quantity of glands is taken out of the phials. The alcohol is allowed to evaporate, if the glands are alcohol preserved ones. Acetone-dried glands are straight away taken from the phials for maceration.
The glands are then macerated in a tissue homogeniser by adding a measured quantity of distilled water or common salt solution or any physiological solution which is isotonic with the blood of the recipient fish. The most successful results of induced breeding in the Indian major carps have so far been obtained with distilled water and 0.3% common salt solution. The concentration of the extract is usually kept in the range of 1-4 mg of gland per 0.1 ml of the media i.e., at the rate of 20-30 gm. of the gland in 1.0 ml of the media. After homogenation, the suspension is transferred into a centrifuge tube. While transferring, the homogenate should be shaken well so that settled down gland particles being mixed with the solution come into the centrifuge tube. The extract in the tube is centrifuged and the supernatent fluid is drawn into a hypodermic syringe for injection.
The pituitary extract can also be prepared in bulk and preserved in glycerine (1 part of extract : 2 parts of glycerine) before the fish breeding season so that the botheration of preparing extract every time before injection is avoided. The stock extract should always be stored in a refrigerator or in ice.
Technique of Breeding:
The induced breeding operation of major carps is taken up when regular monsoon sets in, the fishes become fully ripe and water temperature goes down. Females having a round, soft and bulging abdomen with swollen reddish vent and males with freely oozing milt are selected for breeding. A male breeder can also be easily distinguished by roughness on the dorsal surface of its pectoral fins.
1. Dosage of pituitary extract :
The most important aspect of induced breeding of fish is the assessment of proper dosages of pituitary extract. The potency of the gland varies according to the size and stages of sexual development of the donor, as well as the species of the donor fish, time of collection of glands and their proper preservation. The dose of the pituitary gland is calculated in relation to the weight of the breeders to be injected. It has also been noticed that identical doses to breeders of similar weights may give contradictory results owing to difference in maturity of gonads. Even heavy doses of hormones may not be effective if the gonads are in the resorption stage. By careful selection of breeders and administering a known weight of pituitary gland extract per kg body weight of the breeders, successful breeding can be obtained.
Experiments on standardisation of doses indicate that administration of a preliminary low dose in the female breeder followed by a higher effective dose after 6 hours proves more successful than a single knockout dose. A single high dose has been found useful when the breeders are in ideal condition and the weather is favourable. Rohu responds well to two injections while catla and mrigal to both one and two injections.
An initial dose at the rate of 2-3 mg. of pituitary gland per kg body weight of fish is administered to the female breeder only. Male breeders do not require any initial dose, if they ooze milt on slight pressure on their abdomen. Two males against each female make a breeding set. To make a good matching set, the weight of the males together should be equal to or more than the female. In case the condition of any one of the two males is not found in the freely oozing stage, an initial injection may be administered to the male at the rate of 2-3 mg/ kg body weight. After 6 hours, a second dose of 5-8 mg/kg body weight is given to the female, while both the males receive the first or second dose at the rate of 2-3 mg/kg body weight. Slight alterations in doses may be made depending upon the condition of maturity of the breeders and the prevailing environmental factors. In the absence of a chemical balance, 1-3 pituitary glands are effective for a pair of fish.
2. Method of injection:
Intra-cranial injections are preferred in USSR and intra-peritoneal in USA and Japan. Intra-muscular injection is the most common practice in India. The intra-muscular injection is less risky in comparison with the other mehtods. Intra-peritonial injections are usually given through the soft regions of the body, generally at the base of the pelvic fin or sometimes at the base of the pectoral fin. But there is some risk of damaging the internal organs, specially the distended gonads when administering an intra-peritonial injection in fully mature fishes.
Injections are usually given at the caudal peduncle or shoulder regions near the base of the dorsal fin. While giving injections to the carps, the needle is inserted under a scale keeping it parallel to the body of the fish at first and then pierced into the muscle at an angle. There is no hard and fast rule regarding the time of injection. Injections can be given at any time of the day and night. But since low temperature is helpful and the night time remains comparatively quieter, the injections are generally given in the late afternoon or evening hours with timings
Seed Production Technologies 33
so adjusted that the fish is able to use the quietude of the night for undisturbed spawning.
The most convenient hypodermic syringe used for the purpose is a 2 cc syringe having graduations of 0.1 cc division. The size of the needle for the syringe depends upon the size of the breeders to be injected. No. 22 needle is conveniently used for 1-3 kg carps, No. 19 for larger carps and No. 24 can be used for smaller carps.
Use of anesthetics during injection would significantly increase the survival of brood fish. Commonly used anesthetics are MS 222 and Quinaldine. MS 222 may be added to water in doses of 50-100 mg/ litre. A roll of cotton soaked in a 0.04 M of this solution can be inserted into the mouth of the fish. Quinaldine is used at the rate of 50-100 mg/ litre.
3. Breeding hapa and spawning:
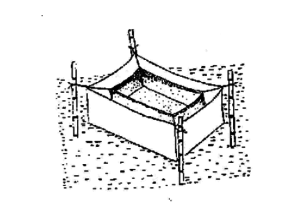
After the injection, the breeders are released immediately inside the breeding hapa. A breeding hapa is generally made of fine cloth in the size of 3.5 x 1.5 x 1.0 m for larger breeders and 2.5 x 1.2 x 1.0 m for breeders weighing less than 3 kg. All the sides of the breeding hapa are stitched and closed excepting a portion at the top for introducing the breeders inside. Generally, one set of breeders is released inside each breeding hapa, but sometimes, in order to save on pituitary material, community breeding is also tried by reducing the number of male breeders. After the release of the fish, the opening of the hapa is securely closed so that breeders may not jump out and escape. Instead of hapas, cement cisterns or plastic pools as big as hapas can also be used for breeding.
Spawning normally occurs within 3-6 hours after the second injection. Soon after fertilisation, the eggs swell up considerably owing to absorption of water. Fertilised eggs of major carps appear like shining glass beads of crystal clear transparency while the unfertilised ones look opaque and whitish. The size of eggs from the same species of different breeders varies considerably. Fully swollen eggs of the Indian major carps measure 2.5 mm in diameter, the largest being that of catla and the smallest of rohu. The carp eggs are non-floating and non-adhesive type. The yolk possesses no oil globule. The Indian major carps have a profuse egg laying capacity. Their fecundity, on an average, is 3.1 lakh in rohu, 1-3 lakh in catla and 1.5 lakh in mrigal.
The developing eggs are retained in the breeding hapa undisturbed for a period of at least 4-5 hours after spawning to allow the eggs to get properly water-hardened. After this, the eggs are collected from the hapa using a mug and transferred into a bucket with a small amount of water. The breeders are then taken out and weighed to find out the difference before and after spawning. This gives an idea of the quantity of the eggs laid. The total volume and number of eggs can be easily calculated from the known volume and the number of eggs of the sample mug. Percentage of fertilised eggs is also assessed accordingly by conducting random sampling before and after spawning. This gives an idea of the quantity of the eggs laid. The total volume and number of eggs can be easily calculated from the known volume and the number of eggs of the sample mug. Percentage of fertilised eggs is also assessed accordingly by carrying out random sampling.
4. Stripping:
Chinese carps however do not spawn naturally and when they spawn, the percentage of fertilisation is generally very low. Stripping (Fig. 3.1) or artificial insemination is therefore followed. The female fish is held with its head slanting upwards and tail down and belly facing the vessel, and the eggs are collected into an enamel or plastic trough by pressing the body of the female. The male fish is then similarly held and milt is squeezed out into the same trough. The gamets are then mixed as soon as possible by means of a quill feather to allow fertilisation. The fertilised eggs are then washed a few times with clean water to remove excess milt and allowed to stay undisturbed in freshwater for about 30 minutes. The eggs are then ready for release into the hatching tanks.
Technique of hatching the eggs:
The eggs collected from breeding hapas are transferred into the hatching hapas. A hatching hapa consists of two separate pieces of hapas, the outer hapa and the inner hapa. The inner hapa is smaller in size and is fitted inside the outer hapa. The outer hapa is made up of a thin cloth in the standard size of 2 x 1 x 1 m while the inner hapa is made of round meshed mosquito net cloth in the dimension of 1.75 x 0.75 x 0.5 m. All the corners of the outer and inner hapas are provided with loops and ropes to facilitate installation. About 75,000 to 1,00,000 eggs are uniformly spread inside each inner hapa. The eggs hatch out in 14-20 hours at a temperature range of 24-310 C. The period of incubation, in fact, is inversely proportional to the temperature. After hatching, the hatchlings escape into the outer hapa through the meshes of the inner hapa. The inner hapa containing the egg shells and the dead eggs which are removed when the hatching is complete. The hatchlings remain in outer hapa undisturbed till the third day after hatching. During this period, they subsist on the food stored up in their yolk sac. By the third day the mouth is formed and the hatchlings begin directive movement and feeding. At this stage they are carefully collected from the outer hatching hapa and stocked into prepared nurseries.
It has been found that Indian major carps could be induced to spawn twice in the same season with an interval of two months. The breeders after the first spawning are fed with groundnut oilcake and rice-bran in the ratio 1:1 at 2.5 percent of the body weight. When favourable climatic conditions occur, they mature and are ready for spawning.
Induced Breeding with H.C.G.
Today pituitary gland extraction is a well established technique for induced breeding all over the world. Its large scale use poses the following problems with regard to availability and quality of pituitary gland (P.G). Inadequate supply of P.G., high cost, variability in pituitary gonadotropin potency and cheating by unscrupulous P.G. suppliers. To overcome these problems, Human Chorionic Gonadotropin (H.C.G) has been found as an alternative for pituitary gland. H.C.G. was discovered in beginning of 1927 by Aschheim and Zondek. They extracted good quality hormone with luteinising gonadotrophic activity from the urine of pregnant women. Russian workers first used chorionic gonadotropin in 1964 with a trade name as Choriogohin and got good results on Loach. Bratanor (1963) and Gerbilski (1965) used H.C.G on carps and trouts and achieved great success. Tang (1968) stated that when Chinese carps were treated with fish pituitary in combination with C.G., effectiveness on induced breeding increased. A perusal of literature indicates that H.C.G. is effective either alone or in combination with P.G. extract in inducing various fishes all over the world.
H.C.G. is a glyco-protein or sialo-protein, because of the carbohydrate molecules attached to the protein molecules. Its primary function is to maintain the production of oestrogen and progesterone by the corpus luteum. It is produced by the placenta and excreted through the urine during early stages of pregnancy (2-4 months). H.C.G comprises of 2 sub-units a and b and has a molecular size of 45,000-50,000 daltons. There are 17 amino acids in it, out of which alanine, proline, serine, cystine and histidine are important. Due to the large number of amino acids, H.C.G. has a high protein content. The molecular weight has been reported as 59,000 by gel filtration and 47,000 by sedimentation equilibrium.
During early stages of pregnancy H.C.G. is rich in the urine of pregnant women. Several methods are employed for the extraction of H.C.G. Aschheim and Zondek (1927) used ethanol for precipitation. Katzman and Caina used different absorbents. Commercial crude H.C.G extraction is made with gel filtration.
Follicle stimulating hormone (FSH) and luteinising hormone (LH) of the pituitary play an important role in the normal reproduction of fish i.e., in promoting the development of gonads, growth, maturity and spawning. H.C.G is more or less similar in character and function to F.S.H and L.H. As pituitary gland is used for induced fish breeding, H.C.G can also be used for early ripening of gonads. Superiority of H.C.G over P.G can be measured on the following grounds. Fish attains maturity faster with H.C.G ., the spawn of the breeding season can be increased with H.C.G ., H.C.G. ensures better survival of spawn, it reduces the time gap between preparatory and final doses, H.C.G is more economical and has a long shelf life, H.C.G is easily available from a standard source, hence is more reliable, periodical injections of H.C.G throughout the year ensure better health and increase in weight and gonadal development Potency of H.C.G is known (30 IU/ mg), available in neat packets of known weights, no preservation is involved, cannot be spurious, H.C.G treated fishes can be used more than once for induced breeding in the same season, mortality rate of hatchlings is negligible, consumption of the drug is less during induced breedings, H.C.G can be used as growth hormone and absorption of eggs at the end of the breeding season is comparatively less by the administration of H.C.G.
The crude H.C.G is in powder form and greyish white or light yellow in colour. It dissolves easily in water. The calculated quantity of crude H.C.G is taken into a tissue homogeniser and stirred for 5-10 minutes with measured distilled water. It is centrifuged for 3-5 minutes. The clear light yellowish supernant liquid having the H.C.G hormones is taken and injected immediately. Any delay in use will result in the loss of the potency.
In case of silver carp (Hypophthalmichthyes molitrix), use of H.C.G is found to be quite successful. The dosage is 4-6 mg/kg. body weight of male, and 6-8 mg/kg body weight of first dose and after about 6-7 hours, 10-12 mg/kg body weight of second dose for female which gave good results. Use of only H.C.G in the breeding of Indian major carps has not given successful results so far. A combination of 60-80% H.C.G and 40-20% P.G for Indian major carps and grasscarps (Ctenopharyngodon idella) is successful. Fishes which are induced to breed with H.C.G alone are mullets, Cyprinus carpio, Lctalurus punctatus, Oreochromis nilotica, Aristichthys nobilis, Misgurnus fossilis, Esox lucius and Epinephelus tauvina.
Recent work shows that the combination of H.C.G and P.G. is more recommendable than H.C.G or P.G alone. More work needs to be done to standardize the dosage of H.C.G for induced breeding of major carps and Chinese carps.
Induced Breeding with Ovaprim
Due to the problem of varying potency of pituitaries, alternatives were tried. Attempts have been made in various countries to use the analogues of luteinizing hormones – releasing hormones (LH-RH) for induced breeding of fishes with varying degrees of success. However, the success achieved with LH-RH was not always consistent, apart from its higher dose requirement for induction of spawning. This epoch making investigation paved the way for developing simple and effective technology for induced breeding of most of the cultivable fishes. In a joint collaborative project, funded by International Development Research Centre, Canada to Dr. Lin of China and Dr. Peter of Canada, a series of investigations were carried out to develop a reliable technology for breeding fishes. Their investigations led to the development of a new technique called as ‘LNPE’ method, wherein an analogue of LH-RH is combined with a dopamine antagonist. Based on the principle, M/s Syndel Laboratories Limited, Canada have manufactured a new drug called as ovaprim.
Ovaprim is a ready to use product and the solution is stable at ambient temperature. It contains an analogue of 20 µg of Salmon gonadotropin releasing hormone (sGnPHa) and a dopamine antagonsist, domperidone at 10 mg/ml. The potency of ovaprim is uniform and contains sGnRHa which is known to be 17 times more potent than LH-RH (Peter, 1987). The dopamine antagonist, domperidone used in ovaprim is also reported to be better than another commonly used antogonist, pimozide. Ovaprim being a ready to use product and one which does not require refrigerated storage, appears to be the most convenient and effective ovulating agent.
This drug is administered to both female and male brood fish simultaneously in a single dose, unlike pituitary extract which is given in two split doses. This reduces not only the handling of brood fish but also helps in saving considerable amount of time and labour which will add on to the cost of seed production. The spawning response in treated species is found to be superior to the pituitary extract injected species.
The efficiency of ovaprim for induced breeding of carps have given highly encouraging results in catla, rohu, mrigal, silver carp, grass carp, big head, etc. The effective dose required for various species of carps is found to vary considerably. The common dose for all carps is 0.10-0.20 ml ovaprim/kg body weight of males and 0.25-0.80 ml ovaprim/kg body weight of females. Female catla is found to respond positively for a dose range of between 0.4-0.5 ml/kg, while rohu and mrigal respond to lower doses of 0.35 ml/kg and 0.25 ml/kg respectively. Among exotic carps, silver carp and grass carp are bred at doses ranging between 0.40-0.60 ml/kg. Big head carp bred successfully at 0.50 ml/ kg. For males of Indian carps, 0.10-0.15 ml/kg and for exotic male carps 0.15-0.20 ml/kg of dosages are found to be optimum. The method of injection is the same as pituitary.
In many countries including our country, ovaprim is used on a large scale for induced breeding of all cultivable fishes successfully. In India, initial trials were conducted during 1988 in Karnataka, Andhra Pradesh and Tamil Nadu.
Ovaprim has unique advantages over pituitary hormone – ready to use liquid form in 10 ml vial, consistent potency and reliable results, long shelf life, and can be stored at room temperature, formulated to prevent over dosing, male and female can be injected only once simultaneously, reduces handling and post breeding mortality, repeated spawning possible later in the season and high percentage of eggs, fertilization and hatching.
Induced breeding with ovatide
Ovatide is an indigenous, cost-effective and new hormonal formulation for induced breeding of fishes. The new formulation is having the base of a synthetic peptide which is structurally related to the naturally occuring hormone, goanadotropin releasing hormone (GnRH). GnRH is not a steroidal hormone and belongs to the class of organic substances called peptides. It is presented as a low viscosity injectable solution which is not only highly active but also cost-effective compared to other commercially available spawning agents. It is also effective in breeding major carps and catfishes. The doses for females are 0.20-0.40 ml/kg for rohu and mrigal, 0.40-0.50 ml/kg for catla, silver carp and grass carp and 0.20-0.30 ml/kg for calbasu. The dosages for males are 0.10-0.20 ml/kg for rohu, mrigal and calbasu, 0.20-0.30 ml/ kg for catla and 0.20-0.25 ml/kg for silver carp and grass carp.
The advantages of ovatide are: It is cost-effective hormonal preparation, it gives high fertilisation and hatching percentage (85-95%), it is increases egg production through complete spawning, it produces healthy seed, it is easy to inject due to its low viscosity, it does not cause adverse effects on brood fish after injection, it can be administered in a single dose to brooders, it can be stored at room temperature, it is quite effective even under climatic adversities and ovatide is available in the market as 10 ml vial, which costs Rs. 300. It is cheaper than ovaprim. The selection of brooders and injecting methods are similar to pituitary extract.
Induced Breeding with Ovopel
Ovopel, developed by the University of Godollo in Hungary, is a preparation containing mammalian GnRH and the water-soluble dopamine receptor antagonist, metoclopramide. The concentration of D-Ala6, Pro9NEt-mGnRH and metoclopramide are in the form of 18-20 micro gm/pellets and 8-10mg/pellets respectively. The hormone is thus available in pellet form. Each pellet contains superactive gonadoptropin releasing hypothalamic hormone analogue with an equal effect which a 3 mg normal acetone-dried dehydrated carp hypophysis gland has. Induced propagation of fish had been shown to be more effective if the hormone was administered in two doses, prime dose and resolving dose, as reported by Szabo, T., 1996. For cyprinids successful results were reported when 2-2.5 pellets/kg were administered to female brood fish. However, preliminary trial with single injection of Ovopel gave encouraging result on a few species of Indian major carps and Clarias batrachus.
The required amount of ovopel was calculated on the basis of weight and condition of brood fish. The pellets were pulverized in a mortar and dissolved in distilled water. The trails were conducted in July-August of 1999.
The new inducing agent. ovopel is easy to store, simple to use and less expensive, as reported by Szabo. T, 1996. However, in India, detailed studies to establish its efficacy and economic viability are required to be undertaken. The hormone has been successfully tested for ovulation in several species of cyprinids, the Common carp, the Silver carp and the tench (Horvath et al, 1997) in Europe. Ovulation was also reported in African Cat fish (Brzuska, E. 1998). In India, Ovopel was used with success in induced breeding of major carps in UP, Haryana and Punjab. In Assam the trials conducted recently on Labeo rohita (Rohu), Cirrihinus mrigala (Mrigal), Labeo gonius (Gonius) and Clarias batrachus (Magur) gave encouraging results. This indicates the possibility of using this new hormone preparation for commercial production of fish seeds if made available to farmers at a competitive price.
Other Substances used for Induced Breeding
Other substances like LH-RH analogues, steroids, and clomiphene are used for induced breeding of fishes.
LH-RH analogue:
Various analogues of Luteinizing hormone -releasing hormone (LH-RH) have been used for induced breeding of fishes. Investigations have revealed that the potential action of releasing hormone when dopamine antagonist is simultaneously used with the analogues is (10-100 µg/kg) used successfully in China. An analogue of teleost GNRH is found to be more potent than LH-RH. GNRH (Gonadotropin releasing hormone) stimulates GTH(Gonadotropin hormone) in teleosts (dosage 25-100 µg/kg).
Steroids:
Selected steroid hormones are used to induce fish. The effects of steroid hormones on ovulation are seen primarily as germinal vesicle breakdown (GVBD). Ovulated oocytes require at least 4 hours to become fertilisable in mullets, whereas in most of the fishes oocytes are fertilisable immediately. The action of pituitary gonadotropins on oocyte maturation is known to be mediated through steroid hormones. Deoxycorticosterone acetate (DOCA) and cortisone effectively stimulate (dosage 50 mg/kg of fish) ovulation in Heteropneustes fossilis (Goswamy and Sunderraj, 1971). 17á-hydroxy-20B dihydroprogesterone (17á-20BDP) is useful to induce gold fish, trout and pikes (Jalabert, 1973). Other steroid hormones commonly used for spawning teleosts are cortisone acetate, deoxycortisol, deoxycorticosterone, hydroxycortisone, progesterone, 11 deoxycorticosterone and 20B progesteron. The advantages of steroids are: most compounds are available as pure preparations in synthetic forms, the quality of steroid preparations is uniform and steroid hormones are much cheaper than gonadotropin preparations.
Clomiphene:
It is an analogue of the synthetic non-steroidal estrogen chlorotrianisene. It is known to have antiestrogenic effects in teleosts. It triggers the release of gonadotropins. The injections of clomiphene (10 µg/g) induced ovulation within 4 days in gold fish, whereas with same dosage, common carp spawned successfully after 40-64 hours.
Estimation of Eggs:
The eggs are collected from the hapa by means of cup or tray or beaker and transferred to the buckets. The breeders are also removed from the hapa and their weights areoted. The difference in weights reveals approximately the number of eggs laid. The eggs are kept in a rectangular piece of close meshed mosquito net and allow the water to drain off. The eggs are measured in a beaker, mug or cup of known volume and transferred to hatcheries. Thus estimation of total quantity is made from total volume of the eggs measured. Percentage of fertilization can be arrived at by counting the number of fertilized eggs from egg samples of 1 ml measure.
Breading of Common carp:
Common carp (Cyprinus carpio) generally breeds in confined water. Spawning takes place in shallow marginal, weed infected areas from January to March and from July to August. Common Carp is also observed to breed round the year. Controlled breeding of common carp is conducted to achieve better spawning and hatching. A set of selected brooders one female and two males are put together in breeding hapa. In order to ensure successful spawning sometimes the female fish is injected with pituitary gland extract at a low dose 2 to 3 mg per kg. Body weight. Freshly washed aquatic weeds (Hydrilla, Najas, Eichhornia etc) are uniformly distributed inside the hapa. These aquatic weeds act as egg collections. The quantity of weed used is roughly double the weight of the female introduced. Each weed attached with 40,000 to 1,00,000 eggs are distributed into a single hatching hapa. After 4 or 5 days the weeds are taken out carefully.
Source: Aquaculture
